CLEANROOM PROJECT
CLEANROOM PROJECT
What’s Clean room?
An area/room which is designed, constructed, used and maintained in such a manner, so as to preclude or reduce the introduction, generation or retention of viable and nonviable particulates. Clean room is regulated and monitored for RH,Temp & ∆P.What’s Quality?
- Meeting the predetermined requirements of the user for a particular product or service.
- Totality of characteristics of an entity that bear on its ability to fulfil implied or stated needs.
What’s GMP?
GMP is that part of QA, which ensures that the products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorization.
A clean room is a rigorously controlled environment that has a low level of environmental pollutants such as dust, airborne microbes, aerosol particles and chemical vapours. The air entering a clean room is filtered and then continuously circulated through high efficiency particulate air (HEPA) and/or ultra-low particulate air (ULPA) filters to remove internally generated contaminants. Staff wearing protective clothing must enter and exit through airlocks, while equipment and furniture inside the clean room is specially designed to produce minimal particles.
Clean room Standard: As per ISO 14644:
Room in which the concentration of airborne particle is controlled, and which is constructed & used in a manner to minimize the introduction, generation, and retention of particles inside the room and in which other relevant parameters (e.g., temperature, humidity, and Pressure) are controlled as necessary.
As per WHO-GMP:An area with defined environmental control of particulate and microbial contamination, constructed and used in such a way as to reduce the introduction, generation, and retention of contaminants within the area.
As per USP Chapter 1116:A room in which the concentration of airborne particles is controlled to meet a specified airborne particulate Cleanliness Class. In addition, the concentration of microorganisms in the environment is monitored; each Cleanliness Class defined is also assigned a microbial level for air, surface and personnel gear
Why Cleanroom?
- Clean, safe and contaminant-free environment is imperative for manufacturing efficacious, safe and good quality drug products or packaging material or allied accessories
- Also critical to protect employees from contact with hazardous materials or pathogens and prevent health problems from prolonged exposure to chemicals or allergens
- Clean rooms are part of GMP requirement and GMP is statutory in nature.
Optimizing a Cleanroom
- Avoiding Contamination / Cleanroom Discipline
- Material Exchange / Air-Showers
- Enclosures for Machinery
- Reducing Power Consumption
- Machines / Process
- Lighting (T8 / T5 / other lamps)
- Work-Flow
- Storage next to end of production line
- Warehouse / Stock centralized
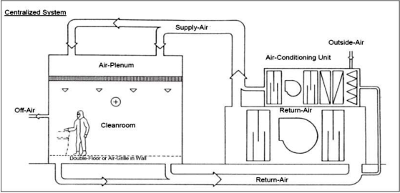
HVAC-Air Handling Concepts & Devices

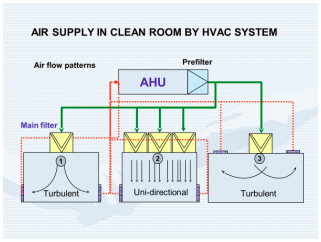
HVAC-Air Handling Concepts & Devices
Optimizing a Cleanroom
